Oral pill Orforglipron delivers stellar results in Phase 3 Trial
Eli Lilly's new drug oral GLP1 Orforglipron delivers fantastic results in phase 3 trial -- 7.6% weight loss for just a pill!
Most GLP1 Receptor Agonists are injected, but there are a few products developed by Novo Nordisk and Eli Lilly (the largest GLP1-focused pharmaceutical companies) that can be taken orally.
Check out our quick explainer
Novo Nordisk created Rybelsus, which has been up until now the only FDA approved oral GLP1, but Eli Lilly has been hard at work on it's own oral GLP1 – Orforglipron:

For the treatment-regimen estimand,4 each dose of orforglipron led to statistically significant A1C reductions. In the key secondary endpoint for body weight, 12 mg and 36 mg doses led to statistically significant reductions.
A1C reduction: 1.2% (3 mg), 1.5% (12 mg), 1.5% (36 mg), 0.4% (placebo)
Percent weight reduction: 4.5% (3 mg), 5.8% (12 mg), 7.6% (36 mg), 1.7% (placebo)
Weight reduction: 4.2 kg (9.3 lbs; 3 mg), 5.2 kg (11.5 lbs; 12 mg), 7.2 kg (15.8 lbs; 36 mg), 1.5 kg (3.4 lbs; placebo)
These results were achieved on a timeline of only 40 weeks – meaning that people lost an extra ~6% body weight with Orforglipron after 40 weeks.
How good were Orforglipron's Phase 3 Trial results?
The results are spectacular. They are on-par with injected semaglutide after 40 weeks, which is quite impressive from a purely oral treatment.
For reference, see the STEP1 clinical trial:

The summary published in the New England Journal of Medicine makes things a bit clearer:
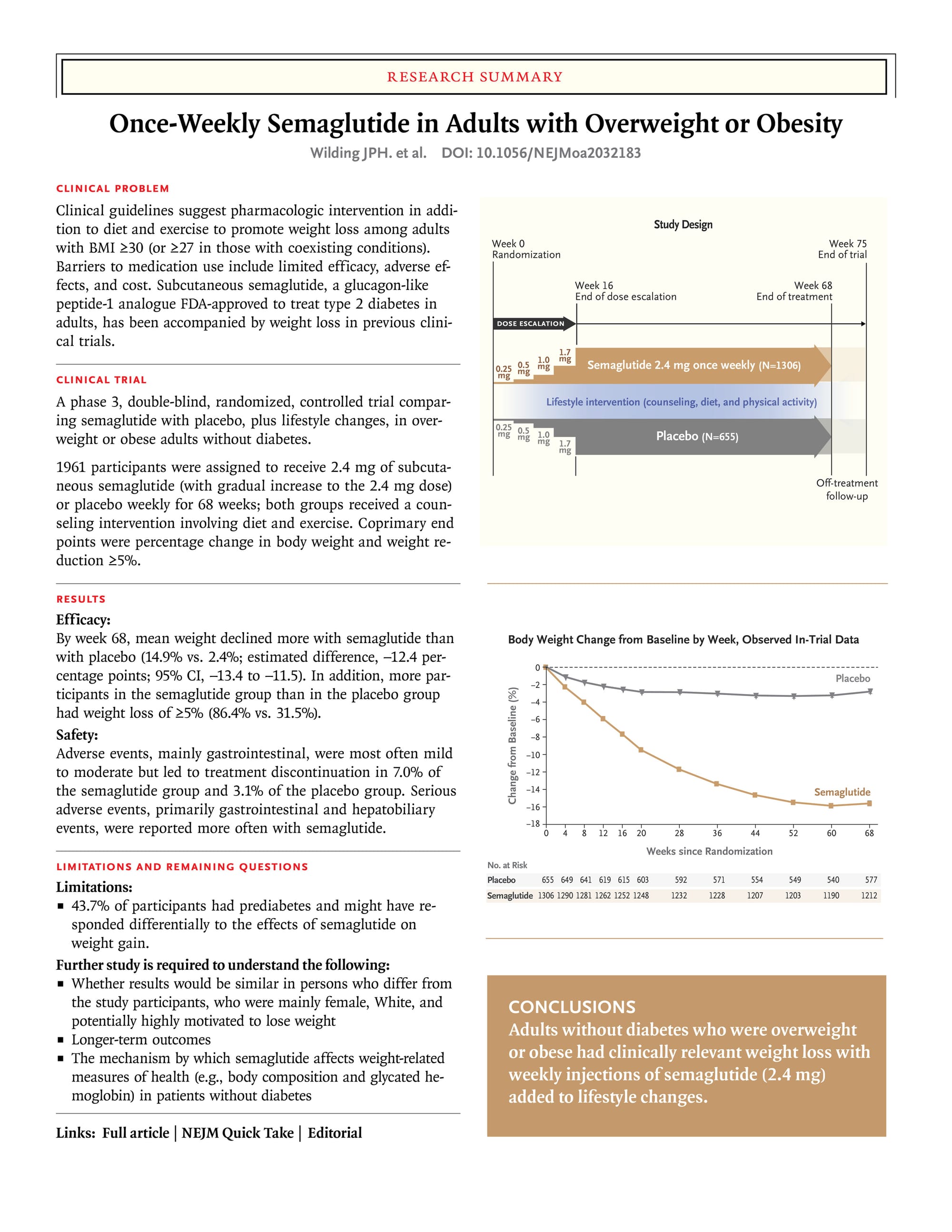
At 44 weeks, placebo weight loss is around 4%, and the loss with semaglutide is around 14%, leaving the differential (i.e. the amount that Semaglutide made a difference) to be around 10%.
At the higher dosages of Orforglipron 40 weeks produced weight loss of 7.6% compared to placebo or 1.7%, which means that Orforglipron boosted weight loss by roughly 5%. Given how inefficient pills are in comparison to injections, this is very impressive.
This result suggests that Orforglipron is around 50% as effective as injected Semaglutide, despite being dramatically easier to take, with no new negative side effects.
To ensure we can trust these results, let's take another look at the study details.
How the study was conducted?
The details of the study are fairly straight forward and look to be high quality (though of course, they are funded by Eli Lilly).
To jump straight to the official (ClinicalTrials.gov) source:
We'll break down some basics below:
- ACHIEVE-1 is a 40-week study with 559 participants (across the US, China, India, Japan and Mexico)
- Randomized, double blind with a placebo control group
- ACHIEVE-1 tests Orforglipron at 3mg, 12mg, and 36mg dosages
- ACHIEVE-1 participants were already having problems with glycemic control given only diet and exercise.
What does this mean for the GLP1 market?
Currently, compounding has been outlawed, and there are only two manufacturers of the highest tier of GLP1s (Semaglutide and Tirzepatide) – Novo Nordisk and Eli Lilly respectively.
This development is great for competition between the two companies, since Novo Nordisk was the only company with an oral treatment on the market – Rybelsus:

That said, Orforglipron is expected to be submitted for FDA approval in 2026 – it won't make it to markets this year, and approval may take time.
In the meantime, this won't do much to increase the supply of GLP1s, but it will certainly be helpful going into the future – more people will be able to easily take a pill rather than injections (and we can avoid producing/requiring the production of injection pens).









