Zepbound coverage for Sleep Apnea gets Agency approval
Center for Medicare and Medicaid declared that Zepbound is covered by insurance for treating Sleep Apnea, given it's effectiveness.

In an interview with CNBC, the Centers for Medicare and Medicaid have declared that Zepbound (Tirzepatide) can now be covered by medicare drug plans:
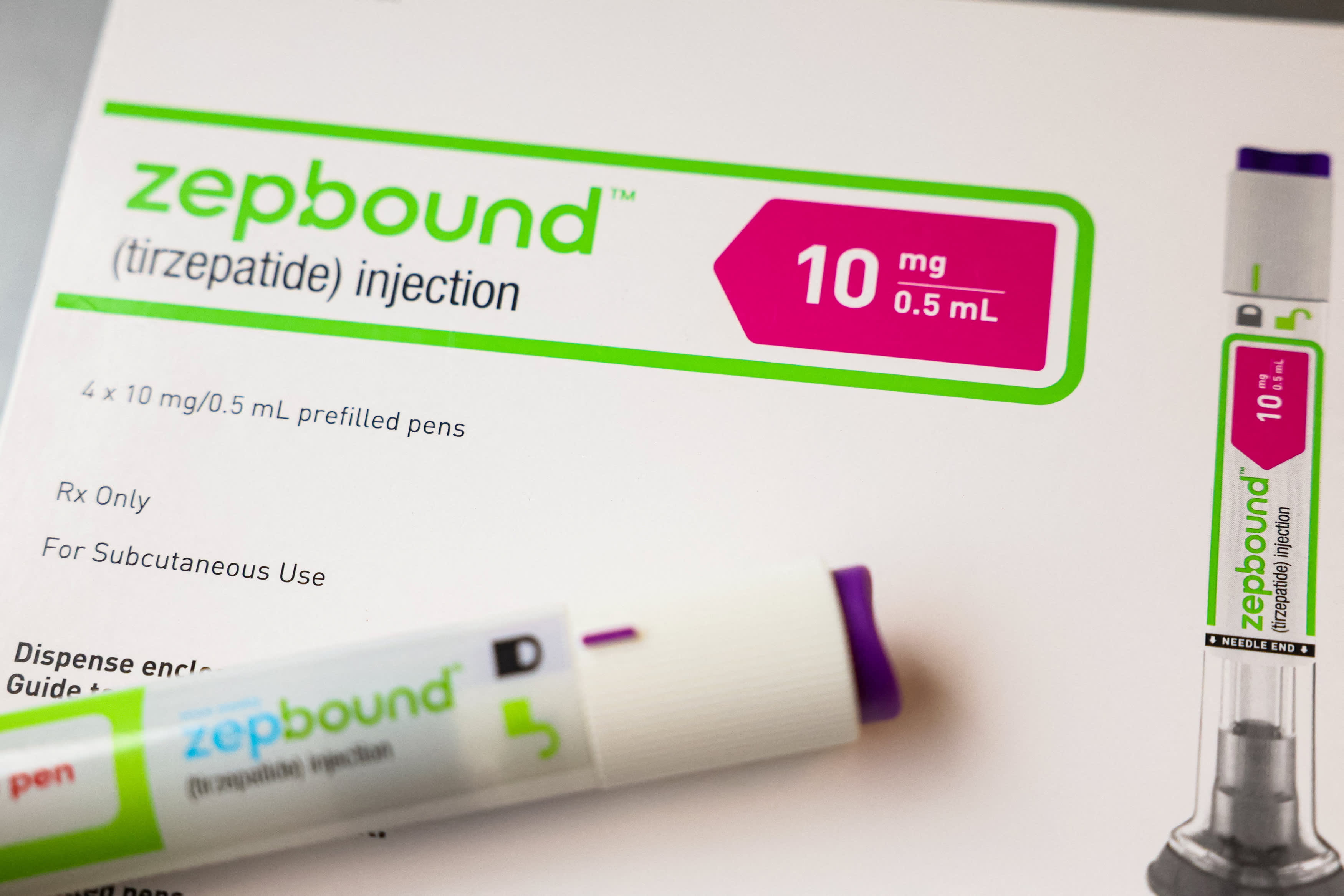
In a statement to CNBC, a spokesperson for the Centers for Medicare & Medicaid Services, an agency of the U.S. Department of Health and Human Services, said "current Medicare Part D and Medicaid coverage rules apply" to Zepbound following its landmark approval in December for the most common sleep-related breathing disorder.
What is Mounjaro/Zepbound (Tirzepatide)? How does it differ from Ozempic (Semaglutide)?
Tirzepatide is a GLP1 Receptor Agonist that was created by Eli Lilly, a pharmaceutical corporation.
Check out our quick explainer
What's unique about Tirzepatide is that it is a dual agonist, both a GIP and GLP1 receptor agonist. While this doesn't translate exactly to being "twice as effective" Tirzepatide has been shown to be more effective than Semaglutide alone:
The rest of this article is no longer available for free – if you'd like to read the rest of our analysis, check us out on Substack: